Learning check
Once you have watched the video, check your learning with this quiz.
Exothermic reactions
 When hydrogen gas is combusted in oxygen (in the air), a lot of energy is released in the form of heat and light. This reaction is thus exothermic.
When hydrogen gas is combusted in oxygen (in the air), a lot of energy is released in the form of heat and light. This reaction is thus exothermic.
Heat (energy) is released.
- Example: Hydrogen is combusted in oxygen.
- 2H2(g) + O2(g) → 2H2O(g) + energy
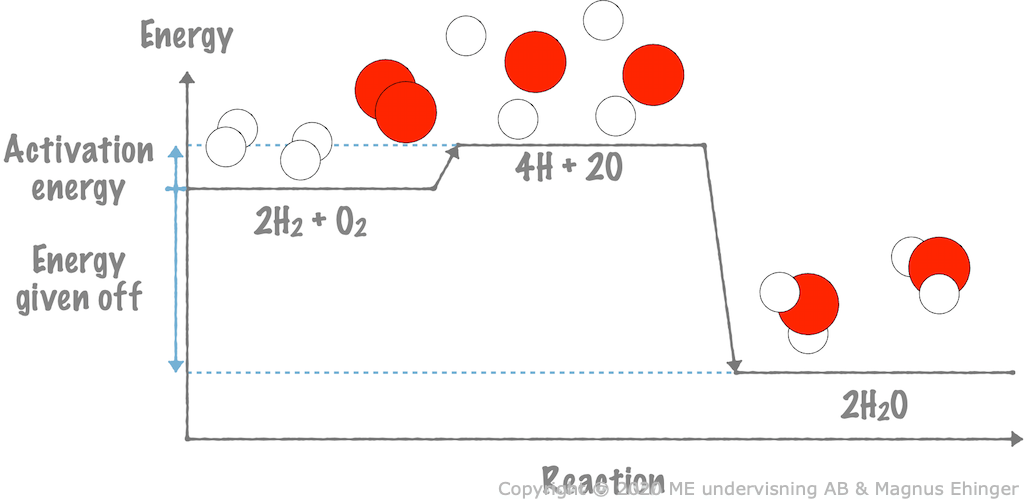 When hydrogen gas reacts with oxygen gas, the activation energy is much smaller than the energy given off.
When hydrogen gas reacts with oxygen gas, the activation energy is much smaller than the energy given off.
To start a reaction, some energy is always required to break the initial bonds.
- Activation energy = the energy required to start a reaction
In exothermic reactions, energy is always released to the surroundings.
Endothermic reactions
Heat (energy) is absorbed.
Example: photosynthesis.
- 6CO2 + 6H2O + light → C6H12O6 + 6O2
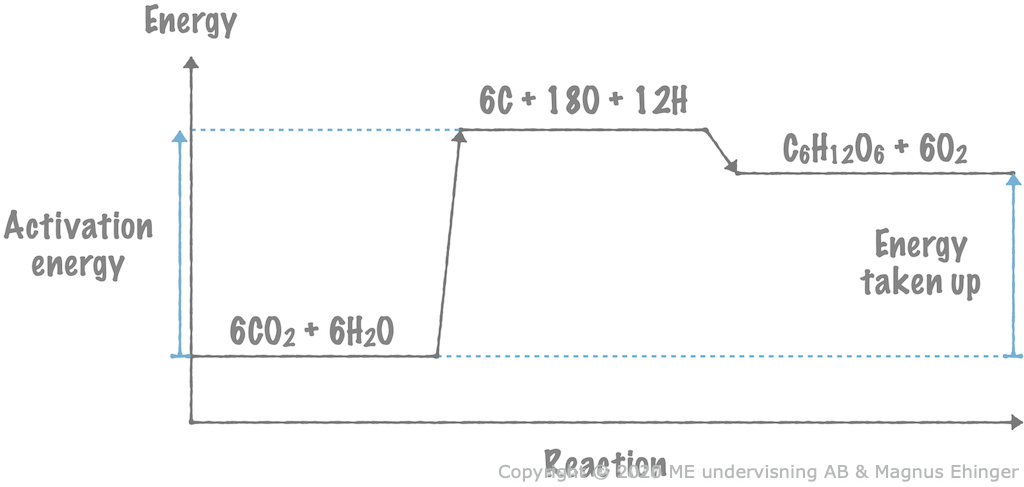 During photosynthesis, the amount of energy given off when new bonds form in glucose and oxygen is much smaller than the activation energy. Thanks to this, the energy from the light is stored in the glucose and oxygen molecules.
During photosynthesis, the amount of energy given off when new bonds form in glucose and oxygen is much smaller than the activation energy. Thanks to this, the energy from the light is stored in the glucose and oxygen molecules.
In endothermic reactions, energy is always taken up from the surroundings.
Two fun reactions
Dissolving sodium hydroxide in water
 When solid sodium hydroxide is dissolved in water, heat is released to the surrounding water, increasing its temperature.
When solid sodium hydroxide is dissolved in water, heat is released to the surrounding water, increasing its temperature.
\({\sf \text{NaOH(s)} \xrightarrow{\text{ H}_2\text{O }} \text{Na}^+\text{(aq)} + \text{OH}^-\text{(aq)} + energy}\)
- We write "+ energy" to the right since energy is released in the reaction.
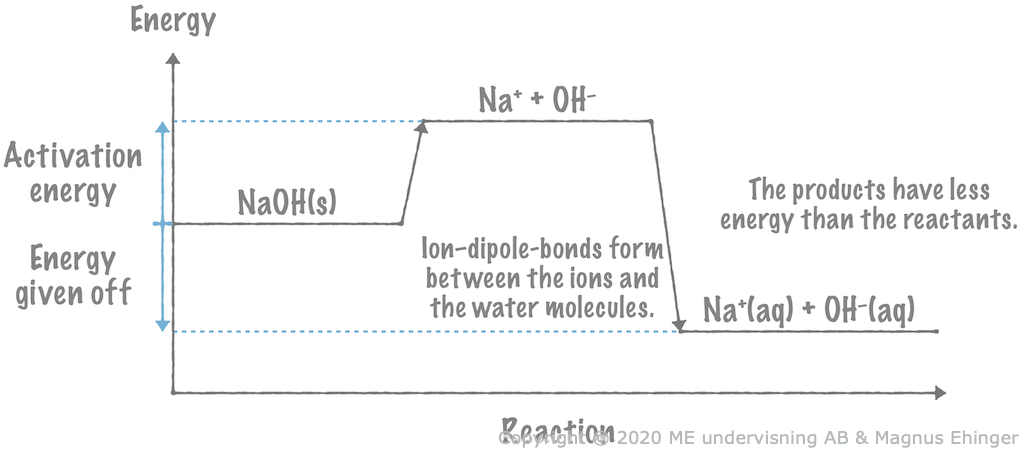 When solid sodium hydroxide is dissolved in water, heat is given off to the surrounding water. This is because the products (the aqueous ions) have less energy than the reactant (solid sodium hydroxide).
When solid sodium hydroxide is dissolved in water, heat is given off to the surrounding water. This is because the products (the aqueous ions) have less energy than the reactant (solid sodium hydroxide).
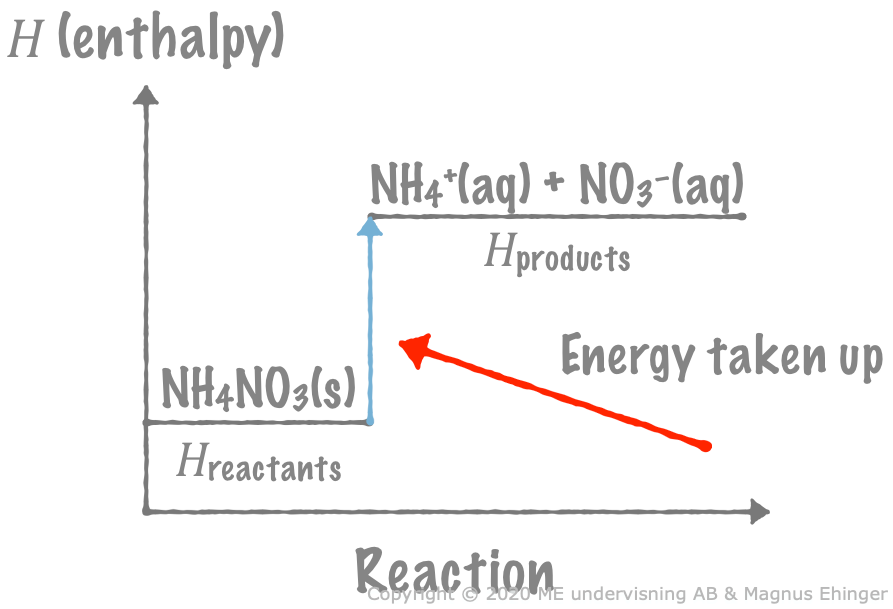
Dissolving ammonium nitrate in water
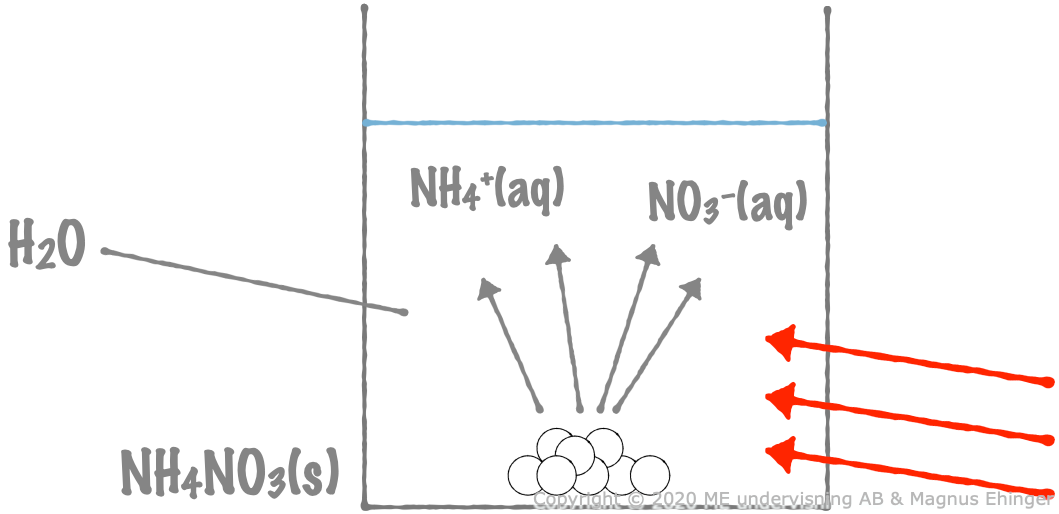 When solid ammonium nitrate is dissolved in water, heat is absorbed from the surrounding water, decreasing its temperature.
When solid ammonium nitrate is dissolved in water, heat is absorbed from the surrounding water, decreasing its temperature.
\({\sf \text{NH}_4\text{NO}_3\text{(s)} + energy \xrightarrow{\text{ H}_2\text{O }} \text{NH}_4^+\text{(aq)} + \text{NO}_3^-\text{(aq)}}\)
- We write "+ energy" to the left since energy is absorbed in the reaction.
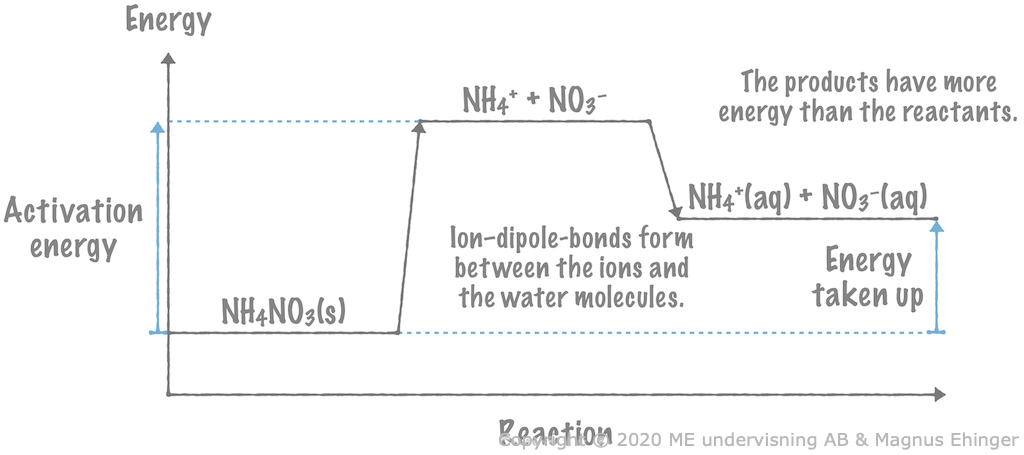 When solid ammonium nitrate is dissolved in water, heat is taken up from the surrounding water. This is because the products (the aqueous ions) have more energy than the reactant (solid ammonium nitrate).
When solid ammonium nitrate is dissolved in water, heat is taken up from the surrounding water. This is because the products (the aqueous ions) have more energy than the reactant (solid ammonium nitrate).
Enthalpy
The energy content of a substance is called its enthalpy.
- Written \(H\)
- Has the unit J (and sometimes J/mol).
In a reaction, the enthalpies for the reacting substances change.
- The change is written \(\Delta H\), and it is equal to \(H_{\text{products}} – H_\text{reactants}\).
An example
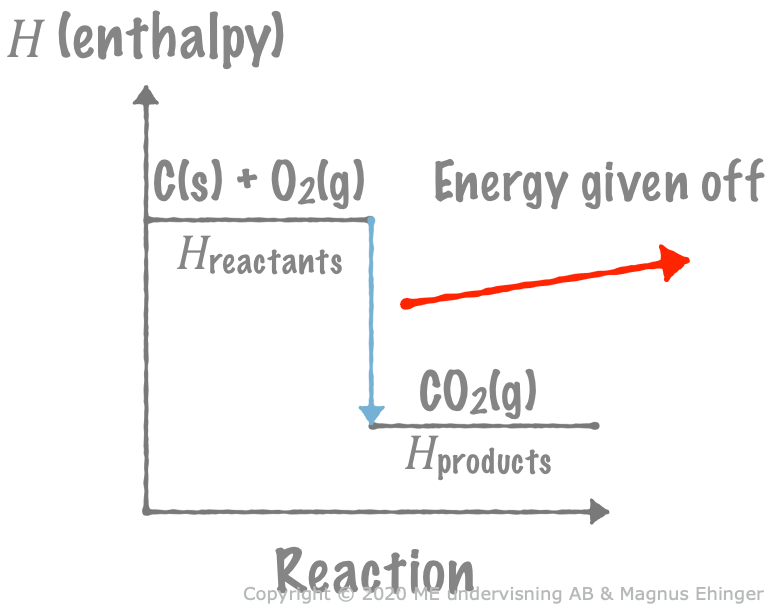
1 mole of carbon reacts with 1 mole of oxygen, forming 1 mole of carbon dioxide.
- C(s) + O2(g) → CO2(g) + 394kJ
What is \(\Delta H\) for the reaction?
Solution
\(\Delta H = H_\text{products} – H_\text{reactants} = H_{\text{CO}_2} - (H_\text{C} + H_{\text{O}_2}) = -394\text{kJ}\)
Why the minus sign?
The sign of \(\Delta H\) for exo- and endothermic reactions
Exothermic reactions: \(\Delta H < 0\)
Endothermic reactions: \(\Delta H > 0\)