Learning check
Once you have watched the video, check your learning with this quiz.
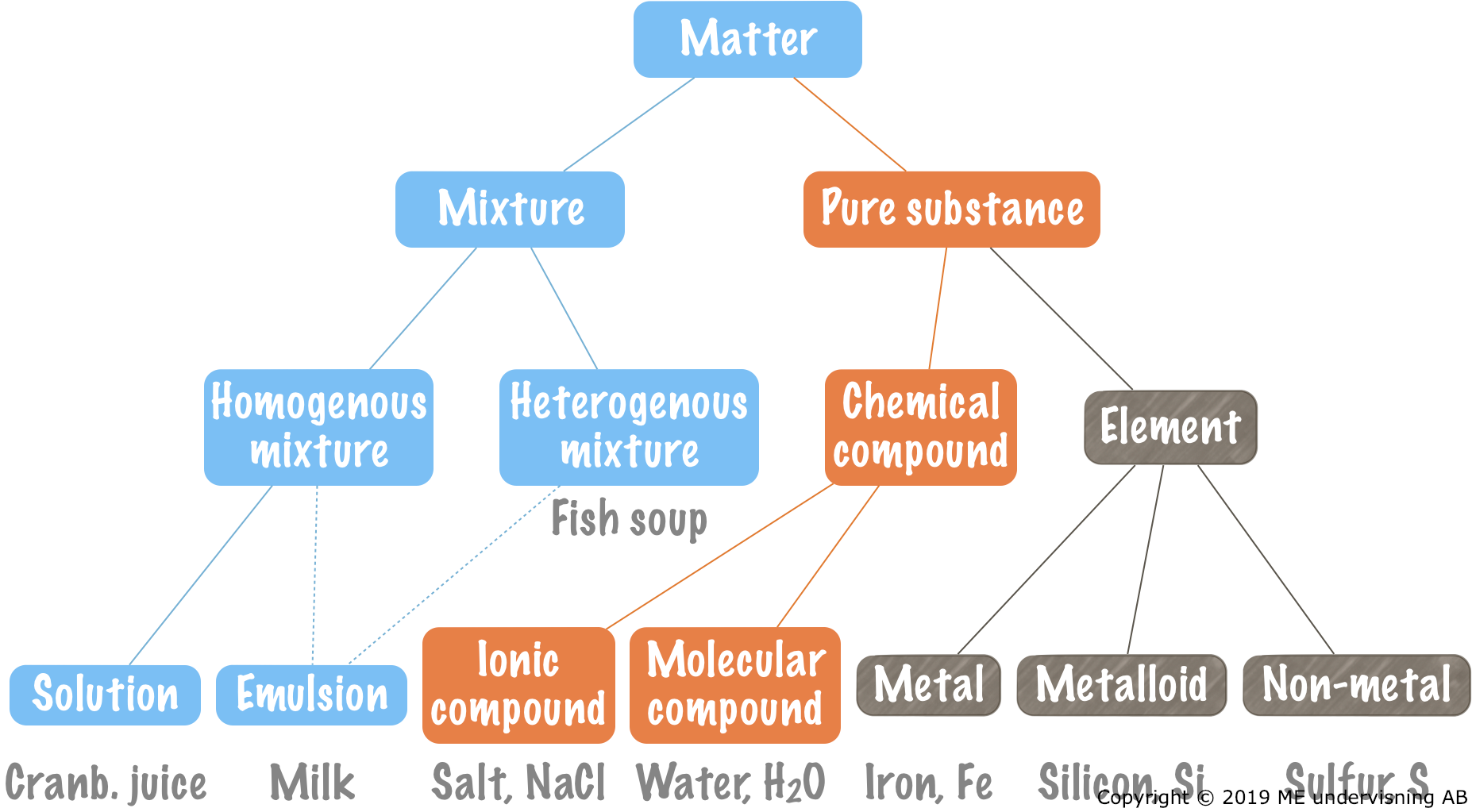
Elements and chemical compounds
An element consists of only type of atom.
Examples:
- Iron, Fe
- Sulfur, S
Mix them and you have nothing more than a mixture of iron and sulfur.
Mixture:
- Two or more substances/chemical compounds included.
- The substances can relatively easy be separated from each other.
- The substances maintain their original properties.
Example:
- Iron is still magnetic.
- Sulfur is still yellow.
Pure substances
Contain only one type of substance/chemical compound.
Examples
- Iron sulfide, FeS
- Water, H2O
- Gold, Au
- Sucrose, C12H22O11
Homogenous and heterogenous mixtures
Homogenous mixtures look the same, all the way through.
May be in gaseous, liquid or solid form.
Examples:
- Air
- Salt dissolved in water
- Bronze
Liquid homomgenous mixtures = solutions
Solid homogenous mixtures = alloys