Learning check
Once you have watched the video, check your learning with this quiz.
Atomic number
Each element has its own number: an atom number.
- The number of protons in the nucleus decides which element it is.
- The atom number = the number of protons.
Examples
| Element | Atomic number | Number of p+ |
| Hydrogen (H) | 1 | 1 |
| Helium (He) | 2 | 2 |
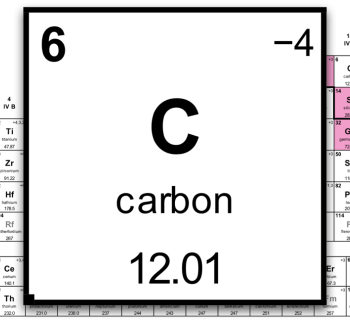
| Carbon (C) | 6 | 6 |
| Chlorine (Cl) | 17 | 17 |
Mass number
Every element (except hydrogen) also contains neutrons.
- Mass number = number of protons + number of neutrons
- \(A = Z + N\)
Two definitions
- Nuclide: An atomic species with a certain number of p+ and n.
- Isotopes: Different nuclides of the same element
 The universe's most simple nuclide.
The universe's most simple nuclide.
Some isotopes and their mass numbers
| Isotope | Nuclide | Atomic nr. | p+ | n | mass number |
| Hydrogen | \( ^1_1\)H | 1 | 1 | 0 | 1 |
| Deuterium | \( ^2_1\)H | 1 | 1 | 1 | 2 |
| Tritium | \( ^3_1\)H | 1 | 1 | 2 | 3 |
| Helium | \( ^4_2\)He | 2 | 2 | 2 | 4 |
| Carbon-12 | \( ^{12}_6\)C | 6 | 6 | 6 | 12 |
| Carbon-13 | \( ^{13}_{\text{ }\text{ }6}\)C | 6 | 6 | 7 | 13 |
| Carbon-14 | \( ^{14}_{\text{ }\text{ }6}\)C | 6 | 6 | 8 | 14 |
| Chlorine-35 | \( ^{35}_{17}\)Cl | 17 | 17 | 18 | 35 |
| Chlorine-37 | \( ^{35}_{17}\)Cl | 17 | 17 | 20 | 37 |
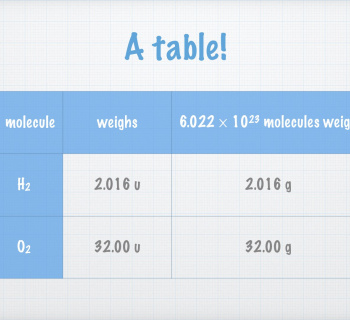
Atomic mass
A single carbon-12 atom weighs 1.994×10–23 g
- Cumbersome figure!
The unified atomic mass unit ”u” (sometimes "amu") has been defined:
- A single carbon-12 atom weighs exactly 12u:
- \(m_{^{12}_{\text{ }\text{ }6}\text{C}}\equiv 12\text{u}\)
Also note: 1u = 1Da
How much do the building blocks of the atom weigh?
- 1 proton weighs approx. 1 u
- 1 neutron weighs approx. 1 u
- 1 electron weighs approx. 1/1800 u
The atomic mass of chlorine
- The atomic mass of chlorine: 35.453u ≈ 35.5u
- There are two chlorine isotopes: chlorine-35 (75 %) and chlorine-37 (25 %)
- The average atomic mass of chlorine:
\[0.75 \times 35\text{u} + 0.25 \times 37\text{u} = 35.5\text{u}\]