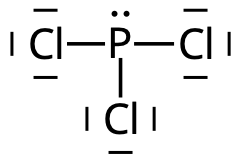Learning check
Once you have watched the video, check your learning with this quiz.
How can you determine a molecule's structure?
"VSEPR" = Valence Shell Electron Pair Repulsion
- Each electron pair in a molecule is repelled by the other pairs.
- Maximal distance between the areas with high electron density ⇒ structure!
Method
- Count the total number of valence electrons + extra charges.
- Divide by two (to get the number of pairs).
- Connect the atoms with single bonds.
- Place electron pairs so that all ligands have noble gas structure.
- Move bonds to the central atom, so that all atoms have noble gas structure.
- Electron pairs repel each other ⇒ conclusion about the molecule’s structure.
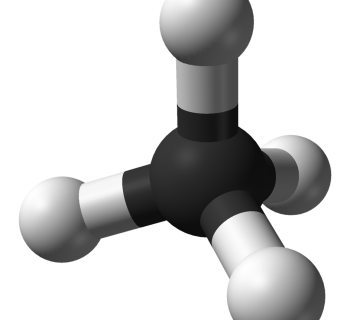
Example 1: Methane
1. Number of valence electrons
- C: 4
- H: 4 × 1 = 4
- In total: 8
2. Divide by two
- Number of electron pairs: 8/2 = 4 pairs
3. Connect the atoms with single bonds
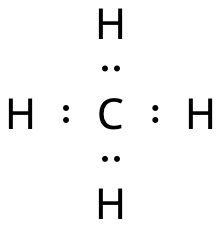
Below, the Lewis dot structure for methane is shown.
4–5. Nobel gas structure + move bonds
The four hydrogen atoms are connected to the carbon atom with covalent bonds.
- All the atoms already have noble gas structure.
6. Conclusion about the molecule's structure
Example 2: Carbon dioxide, CO2
1. Number of valence electrons
- C: 4
- O: 6 × 2 = 12
- Totalt: 16
2. Divide by two
- Number of electron pairs: 16/2 = 8 pairs
3. Connect the atoms with single bonds
The carbon atom is placed centrally, the oxygen atoms are ligands:
4. Place the rest of the valence electrons
The electrons are placed so the ligand atoms get noble gas structure:
5. Move electron pairs
The carbon atom also gets noble gas structure:
6. Conclusion about the molecule's structure
Example 3: Beryllium chloride, BeCl2
1. Number of valence electrons
- Be: 2
- Cl: 7 × 2 st = 14 st
- Totalt: 16
2. Divide by two
- Number of electron pairs: 16/2 = 8 pairs
3. Connect the atoms with single bonds
The beryllium atom is placed centrally, the chlorine atoms are ligands:
4. Place the rest of the valence electrons
The electrons are placed so the ligand atoms get noble gas structure:
5. Move electron pairs
The carbon atom also gets noble gas structure:
6. Conclusion about the molecule's structure
Two areas with high electron density (the double bonds) ⇒ 180° between the bonds.
- Just like the CO2-molecule, BeCl2 is linear.
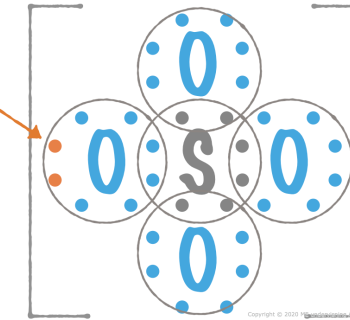
Example 4: Sulfur dioxide, SO2
1. Number of valence electrons
- S: 6
- Cl: 6 × 2 st = 12
- Totalt: 18
2. Divide by two
- Number of electron pairs: 18/2 = 9 pairs
3. Connect the atoms with single bonds
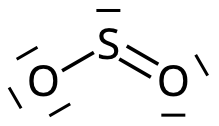
The sulfur atom is placed centrally, the oxygen atoms are ligands:
4. Place the rest of the valence electrons
The electrons are placed so the ligand atoms get noble gas structure.
- Six of the remaining electron pairs are placed on the oxygen atoms.
- The last electron pair is placed on the sulfur atom:
5. Move electron pairs
The sulfur atom also gets noble gas structure:
6. Conclusion about the molecule's structure
Exercises
Use the VSEPR theory to determine the structures of each of the following molecules:
1. Carbon monoxide, CO
What is the structure of the carbon monoxide molecule, CO?
Answer
It is linear.
Lewis structure
2. Dihydrogen sulfide, H2S
What is the structure of the dihydrogen sulfide molecule, H2S?
Answer
It is bent.
Lewis structure
3. Phosphor trichloride, PCl3
What is the structure of the phosfphor trichloride molecule, PCl3?
Answer
It is pyramidal.