Learning check
Once you have watched the video, check your learning with this quiz.
pH and pH indicators
How do you know if something is acid?
- It has a sour taste.
- A pH indicator may show it.
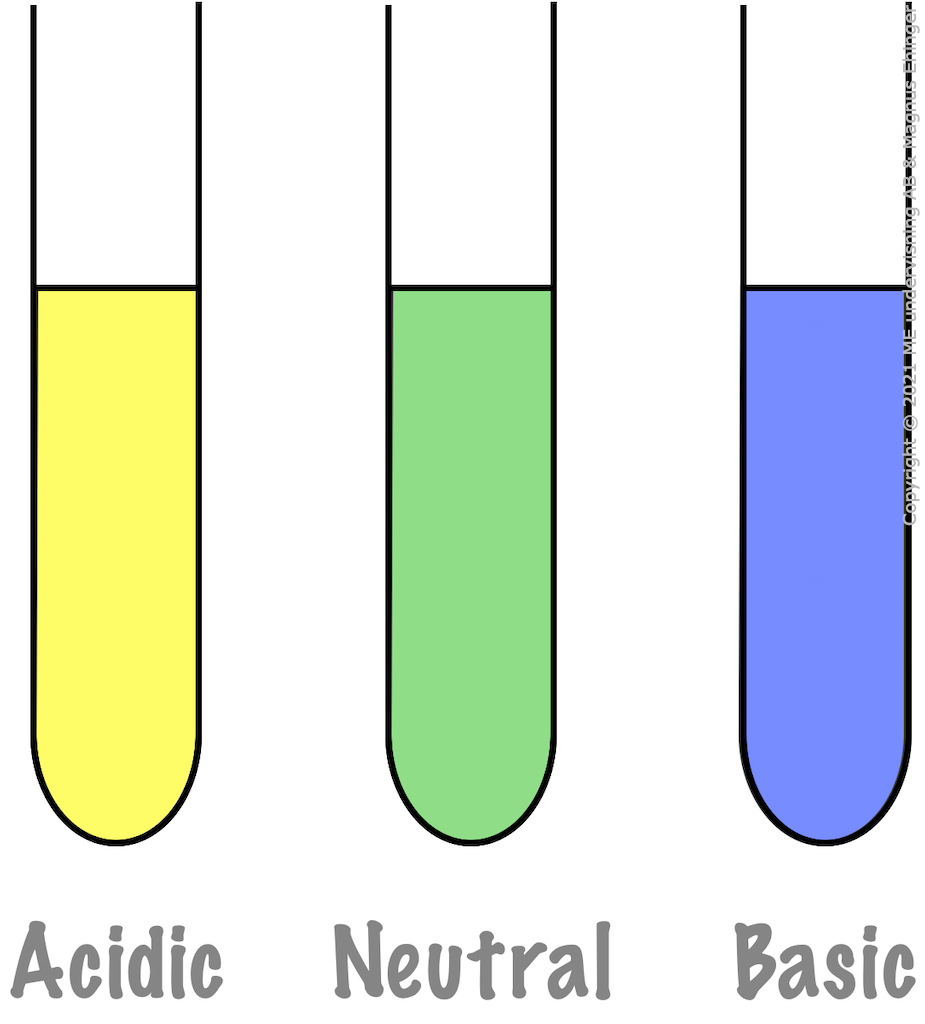
BTB (Bromothymol blue):
- Yellow in acidic solutions.
- Green in neutral solutions.
- Blue in basic (alkaline) solutions.
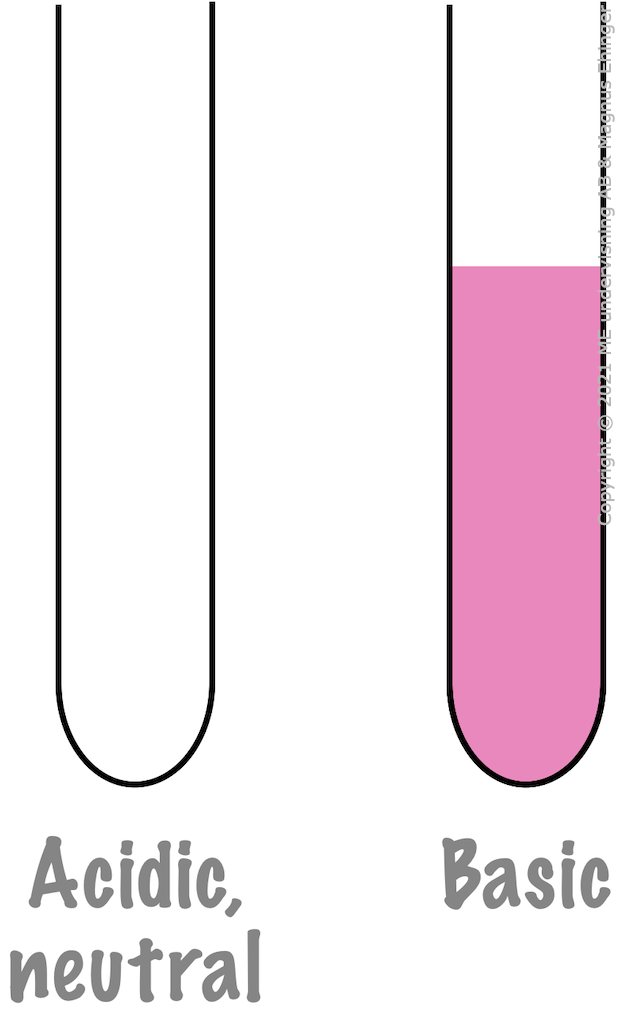
Phenolphthalein.
- Colorless in acidic and neutral solutions.
- Pink in basic solutions.
Acidic and basic (alkaline) solutions
- Acidic solution: pH < 7
- Neutral solution: pH = 7
- Basic solution: pH > 7
Acidic solutions conduct electricity
Acidic solutions conduct electricity because there are ions in the solution.
- The more ions, the higher the conductivity.
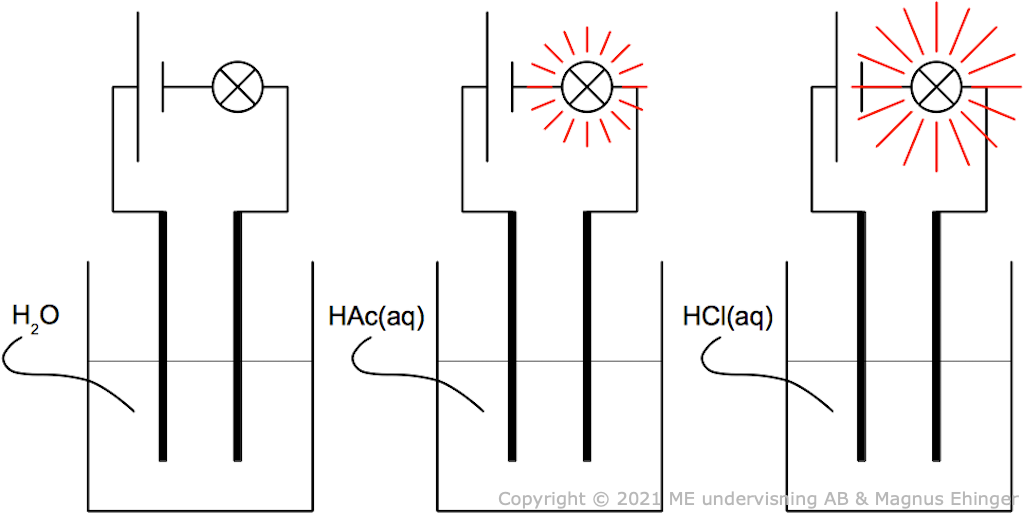 Acidic solutions conduct electricity.
Acidic solutions conduct electricity.
Why are there ions in an acidic solution?
- The hydrolysis (reaction with water) of hydrochloric acid:
 Hydrochloric acid reacts with water. A chloride ion and a hydronium ion form.
Hydrochloric acid reacts with water. A chloride ion and a hydronium ion form.
- Acidic substances donate protons (hydrogen ions, H+).
- Acidic solutions contain hydronium ions (H3O+).
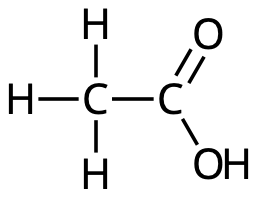
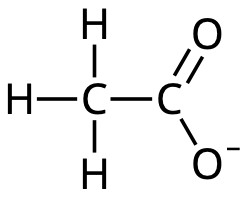
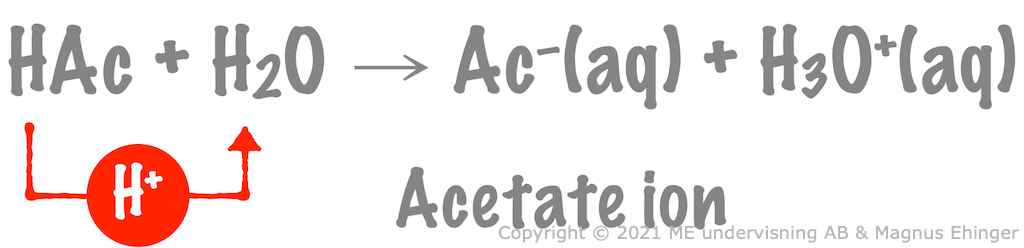
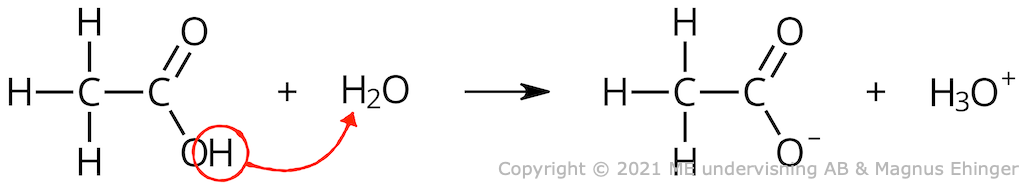
Acetic acid
Protolysis of acetic acid:
Acids easily react with base metals
Example: Magnesium in hydrochloric acid
- Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
- Mg(s) + 2H+(aq) + 2Cl–(aq) → Mg2+(aq) + H2(g) + 2Cl–(aq)
Note: Hydrogen gas is formed.
- Base metals are sometimes called hydrogen-expelling.