Learning check
Once you have watched the video, check your learning with this quiz.
Aluminium is passivated by a very hard and air-tight oxide layer:
4Al + 3O2 → 2Al2O3
Gallium, Ga
Low melting point, 29.8 °C.
Used in LED lamps.
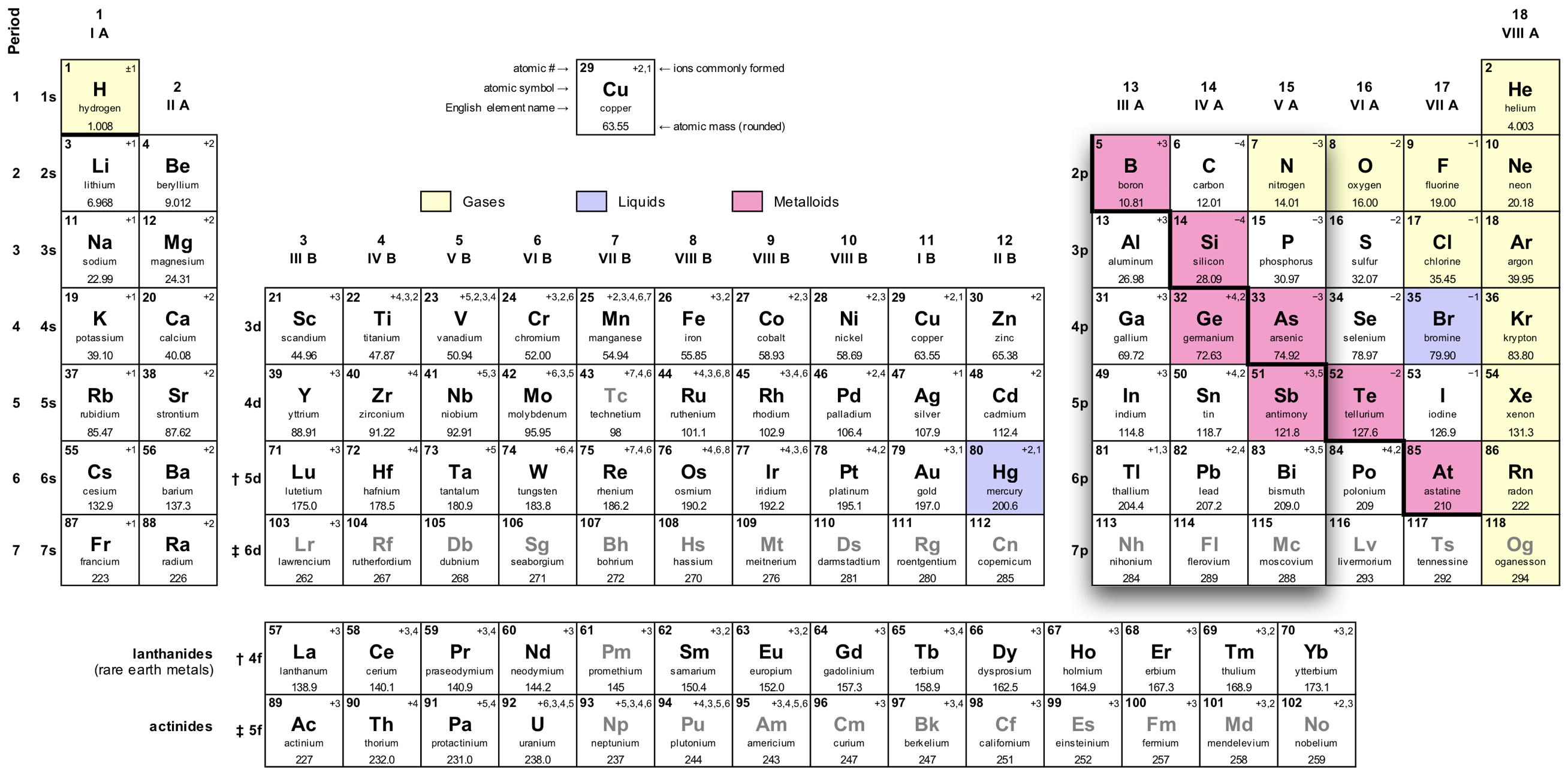
The carbon group
Four valence electrons
Example: The electron configuration for carbon, 6C:
| K | L | |
| 5p+ | 2e– | 4e– |
Carbon in five different forms
Silicon, Si
A metalloid.
Second most common element in the earth’s crust.
Quartz mineral: Silicon dioxide, SiO2.
Used in glass and electronics.
Tin, Sn
Tin is a metal.
Uses:
- Alloy with copper → bronze
- Tin casting
- Tin plating (corrosion protection)
Lead, Pb
Heavy metal, poisonus.
Plumber = someone who works with pipes (made of lead, lat. plumbum).
Uses: Ammunition, protection against radiation (and exclusive glass decanters).
The nitrogen group (pnictogens)
Five valence electrons
Example: The electron configuration for nitrogen, 7N:
| K | L | |
| 5p+ | 2e– | 5e– |
Nitrogen, N
Air; 78 % nitrogen gas, N2(g)
- Inert.
- Reacts with oxygen at high temperatures, nitrogen oxides form.
- Converted to ammonia, NH3, by nitrogen-fixating bacteria.
Important part of DNA and proteins.
Phosphorus, P
Exists in a few different forms, for example:
- White phosphorus, P4
- Red phosphorus in the match boxes’ striking surface
When combusted, forms phosphates, PO\(_4^{3-}\).
Also important part of DNA. Also in phospholipids, which make up biological membranes.
Arsenic, As
Metalloid
Arsenic poisoning (even lethal):
- Arsenic-containing bedrock, deep wells.
- Problem in e.g. Bangladesh!