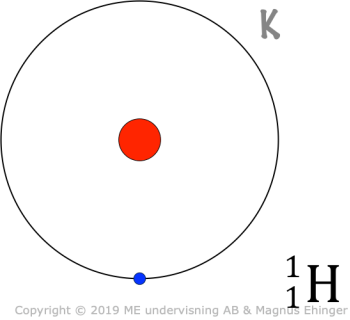
Learning check
Once you have watched the video, check your learning with this quiz.
Which are the noble gases?
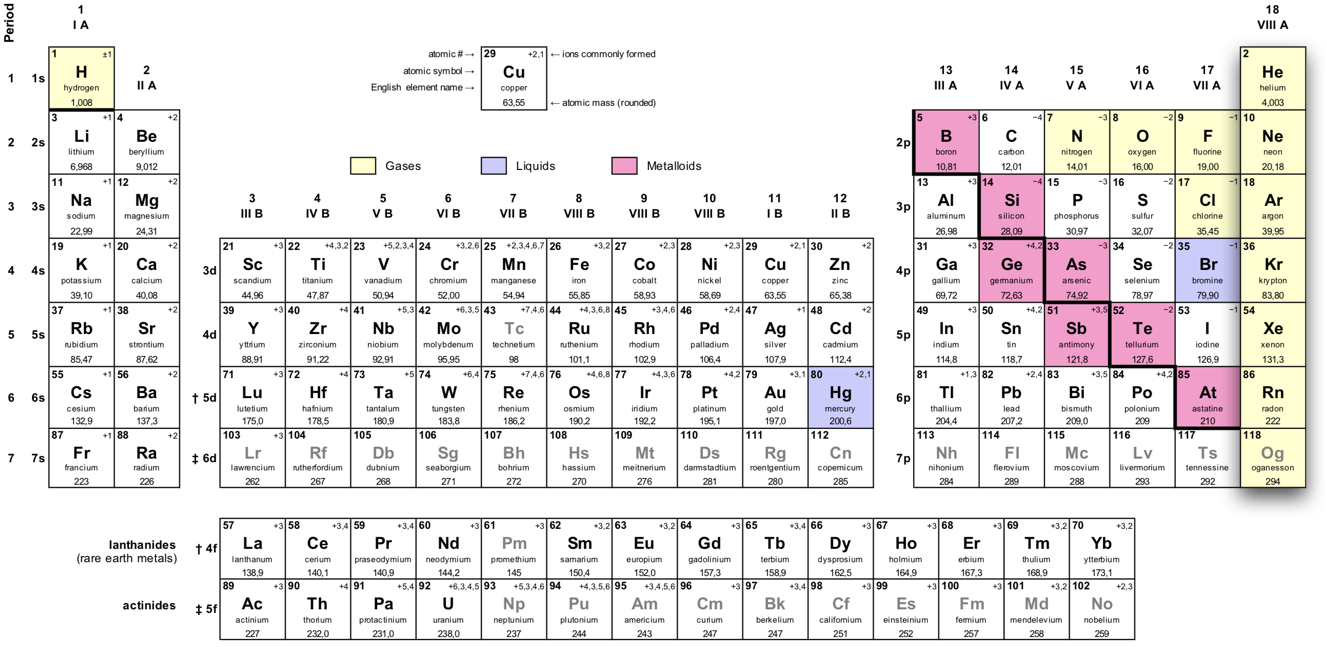 The noble gases' location in the periodic table.
The noble gases' location in the periodic table.
The noble gases are:
- Helium, He
- Neon, Ne
- Argon, Ar
- Krypton, Kr
- Xenon, Xe
- Radon, Rn
- Oganesson, Og
Noble gas structure
All the noble gases have a full valence shell.
- He: 2 valence electrons
- Ne, Ar, Kr, Xe, Rn, Og: 8 valence electrons
Full valence shell = extra stable
- The noble gases ar inert (they don’t react with anything).
- The noble gases are mostly found in their pure (noble) form.
Some common characteristics
- Inert
- Color-, odorless (oganesson: unknown)
- Very low melting points
Some uses
Helium
- Cooling agent (–269 °C)
- ”Fun things”
Neon
- Lights (neon lights)
Argon
- Welding: Prevents the oxygen in the air from reacting with the metal.
- Incandescent bulbs: Atmosphere with Ar + N2, prevents the filament from combusting.
Radon
- Radioactive
- Harmful for smokers.
Oganesson
- The heaviest (and last?) element of the periodic table.
- Characteristics: Highly radioactive, otherwise unknown