Learning check
Once you have watched the video, check your learning with this quiz.
Electron clouds
Electrons are actually not tiny marbles of negatively charged matter.
- The electron is “smeared” in time and space.
- One cannot determine the electron’s speed and its position at the same time.
- One can only determine where an electron is with some specific probability.
Because of this, we talk about elecron clouds; a "cloud" where there is some probability that the electron is.
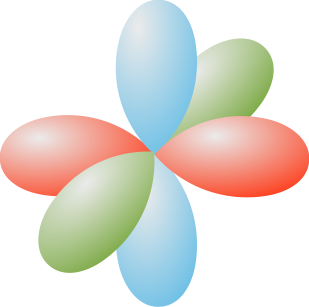
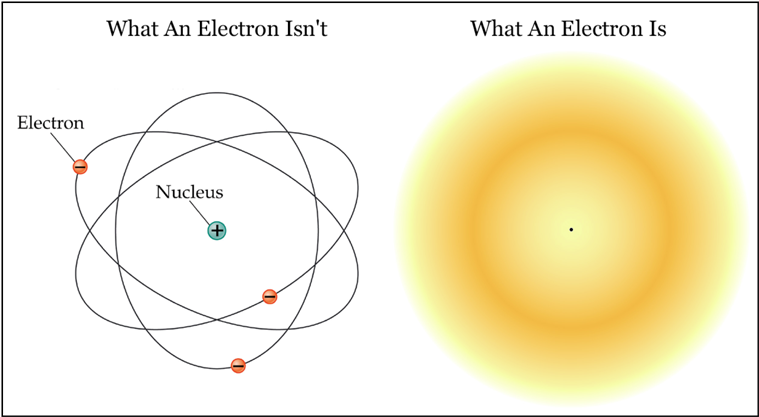 What an electron isn't and what an electron is.
What an electron isn't and what an electron is.
Shells and orbitals
All the electrons in a shell have the same average energy. The electrons may reside in different energy levels (orbitals) within the same shell.
- All the electrons in a certain shell have the same average energy.
- Each orbital may contain max 2e–.
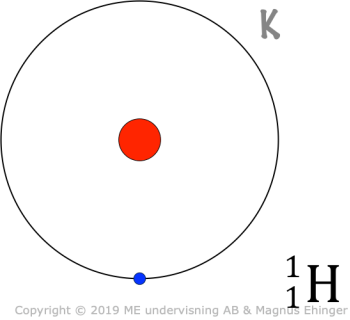
The K shell
Contains a single orbital, 1s.
- Since the K shell only contains one orbital (1s), and each orbital maximally contains 2e–, there can be only two electrons in the K shell.
The L shell
Contains
- One s orbital, 2s
- Spherical
- Maximally 2e–
- Three p orbitals, 2p
- Dumbbell-shaped
- Maximally 3 × 2e– = 6e–
In total: Maximally 8e– in the L shell.
The M and N shells
The M shell has three energy levels:
- One s orbital, 3s
- Three p orbitals, 3p
- Five d orbitals, 3d
The N shell has four energy levels:
- One s orbital, 4s
- Three p orbitals, 4p
- Five d orbitals, 4d
- Seven f orbitals, 4f
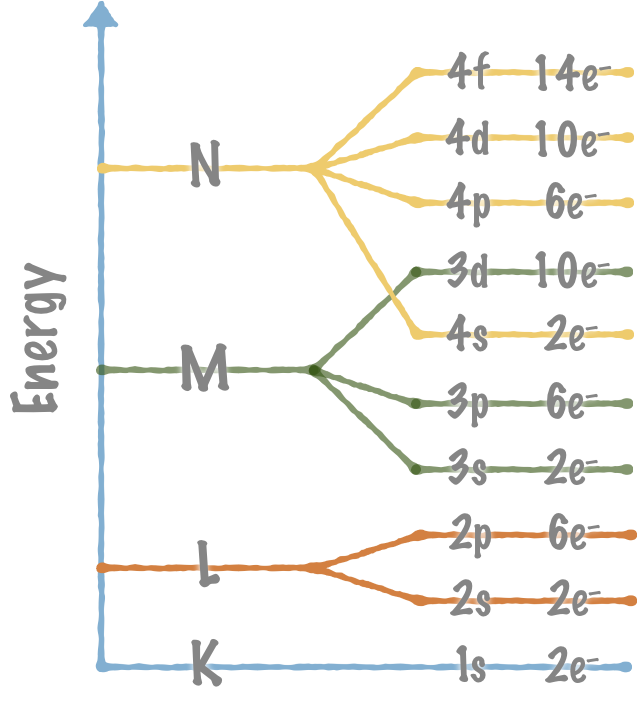
Note: The 3d and 4f orbitals overlap.
- The energy of the electrons in the 4s orbitals are lower than the energy of the electrons in the 3d orbitals.
- The 4f orbitals are filled before the 3d orbitals.
The Madelung rule (the Aufbau principle)
Let's look at the electron configurations for a few atoms.
Argon, 18Ar:
| K | L | M | |
| 18p+ | 2e– | 8e– | 8e– |
Note: The 3s and 3p orbitals in the M shell are completely filled.
In potassium, 19K, the next electron ends up in the 4s orbital of the N shell, because there its energy is lower than in the 3d orbital of the K shell.
- This is why there are never nine electrons in the outermost shell, only eight electrons at most.
The electron configuration for 19K:
| K | L | M | N | |
| 19p+ | 2e– | 8e– | 8e– | 1e– |
Let's also look at the electron configuration for calcium, 20Ca:
| K | L | M | N | |
| 20p+ | 2e– | 8e– | 8e– | 2e– |
In calcium, the N shell's 4s orbital is filled in calcium. The next electron (in scandium, 21Sc) must be placed in one of the M shell's 3d-orbitals:
| K | L | M | N | |
| 21p+ | 2e– | 8e– | 8e– | 2e– |
When the M shell's 3d-orbitals are filled, we can start adding electrons the N shell's 4p orbitals. In gallium, 31Ga, there are 10e– in the M shell's 3d orbitals, and the last electron ends up in the 4p orbital of the N shell. This means there are 3e– in the N shell:
| K | L | M | N | |
| 31p+ | 2e– | 8e– | 18e– | 3e– |
In krypton, 36Kr, the 4p orbitals have been filled up with 6e–, leading to 8e– in the N shell:
| K | L | M | N | |
| 36p+ | 2e– | 8e– | 18e– | 8e– |
The rest of the orbitals are filled in order according to the Madelung rule (the Aufbau principle). The arrows indicate in which order the orbitals are filled:
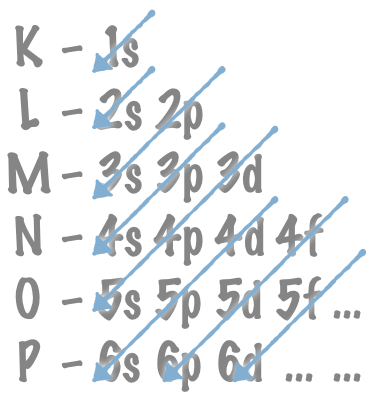 The electron orbitals are filled according to the Madelung rule (the Aufbau principle).
The electron orbitals are filled according to the Madelung rule (the Aufbau principle).
Writing ground states
Using the Madelung rule, we can determine the electron configuration for e.g. argon 18Ar. Each superscripted number indicates the number of electrons in the orbitals:
- 1s2 2s2 2p6 3s2 3p6
Another example, zinc 30Zn:
- 1s2 2s2 2p6 3s2 3p6 4s2 3d10
Since the first five orbitals are identical to 18Ar, we can also write the electron configuration for zinc 30Zn like this:
- [Ar] 3d10 4s2