Learning check
Once you have watched the video, check your learning with this quiz.
The atomic theory
Democritus first suggested that matter may be made of indivisible particles = atoms. John Dalton provided the first scientific atomic theory (based on experiments) supporting the idea of "atoms" (1805).
Dalton's atomic theory stated that:
- Matter is made of solid "marble-like" particles (atoms).
- The mass of the atom decides what the properties of the atoms are.
Thanks to Dalton's new theory, elements can be said to be made of atoms. Jöns Jacob Berzelius compiles a table with the relative masses of the then-known atoms (1818), which are later used by Dmitri Mendeleev to organize the periodic table (1869).
Henri Becquerel
Studies uranium salts (1896).
- Discovers that a photographic plate is exposed by the uranium.
- Not caused by x-rays!
Marie Curie
Characterizes the radiation from e.g. radium (1896).
- Coins the term ”radioactivity”.
- The atom is not indivisible!
Nobel prize in physics 1903 (together with her husband Pierre, and Henri Becquerel).
J. J. Thomson
Uses a cathode ray tube to discover and characterize the electron (1898).
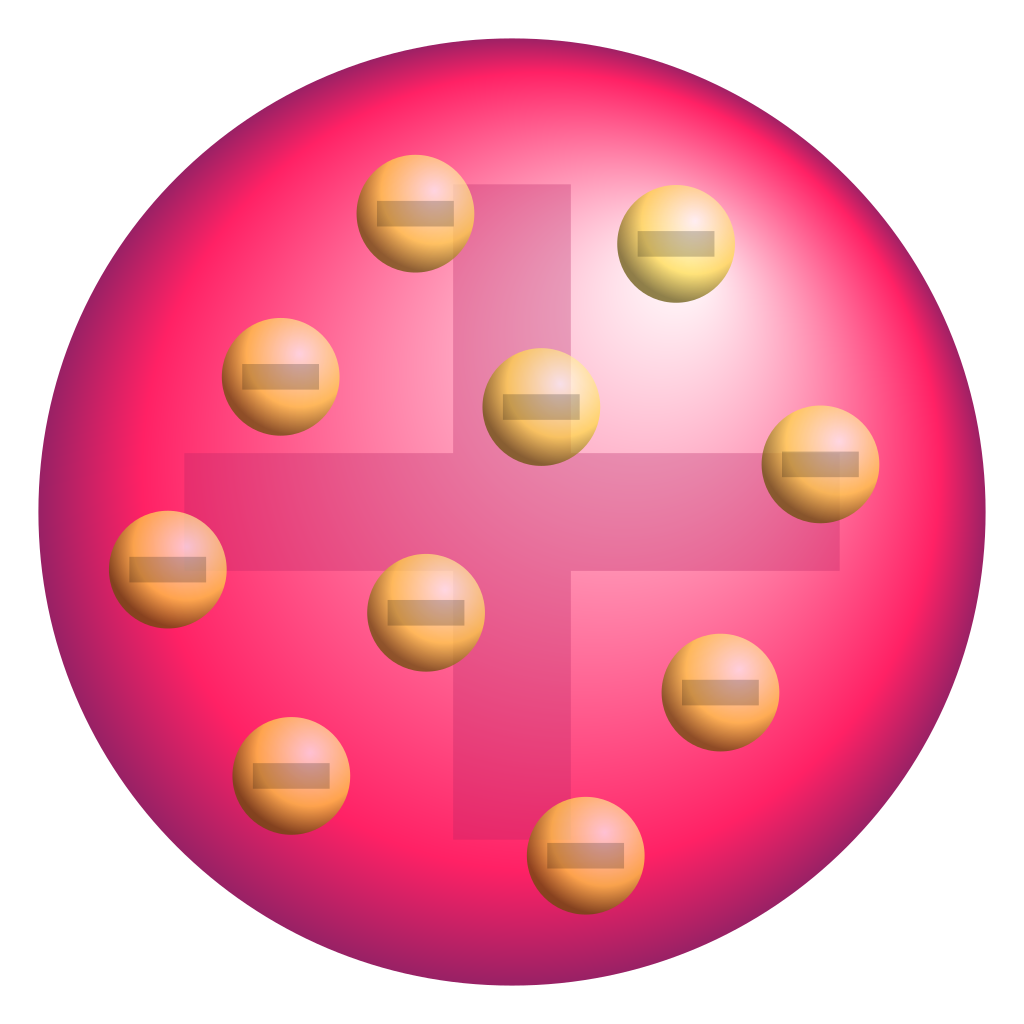
The "plum pudding" model
The electrons are spread throughout the atom, like plums in a plum pudding.
Ernest Rutherford
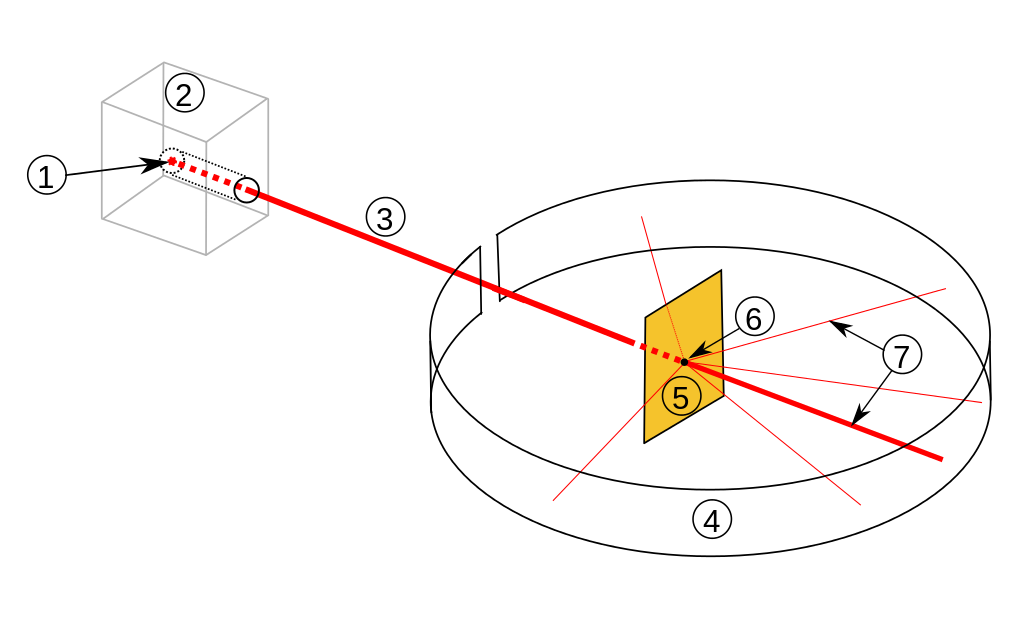
The gold foil experiment
Alpha particles beamed at a thin gold foil.
- Thomsons "plum pudding" model predicted: Particles should pass right through.
- Reality: About 1/8000 particles bounced of a very dense, solid nucleus.
Rutherford's atomic model
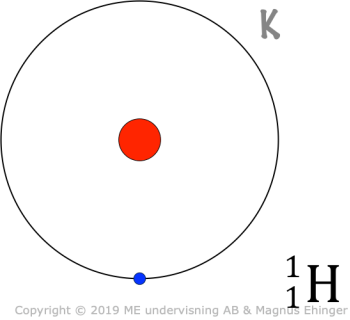
- A very small, dense, and positively charged nucleus.
- Electrons circle around, like ”planets”.
Niels Bohr
Problem with Rutherford's model: The electrons should immediately fall back into the nucleus.
 The visible spectrum of hydrogen.
The visible spectrum of hydrogen.
- Bohr studied the emission spectrum from hydrogen.
- Only certain colors (energies) are emitted ⇒ The electrons may only reside in certain energy levels (electron "shells").
Beyond Bohr's model
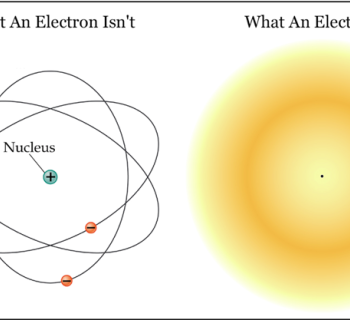
- In reality, the electron shells are not that simple.
- The electrons are not negatively charged ”marbles”, but rather ”smeared” in time and space.
- Electron clouds rather than electron shells.
Protons and neutrons
Protons discovered by Rutherford (1917).
- Problem: How can the protons stay in the nucleus?
- There must be some kind of ”glue”!
Neutrons discovered by Rutherford's student, James Chadwick (1932).
Summary: The building blocks of the atom
Electrons
- Charge: –1 (e–)
- In different energy levels (shells) around the nucleus
Protons
- Charge: +1 (p+)
- In the nucleus
Neutrons
- Charge: 0 (n)
- In the nucleus